What is Mass Number – Friends, today we will learn about mass number, what is mass number and what is its definition and why mass number is used in chemistry and we will give you many more information about mass number. You will know about mass number in this post. You will also learn about the examples so that you can know the mass number in a better way.
So you must keep reading this post till further, after this you will get complete information about Mass numbers. You will get complete information What is Mass Number about Mass numbers. Mass numbers are always useful in chemistry and Mass.
What is Mass Numbers?
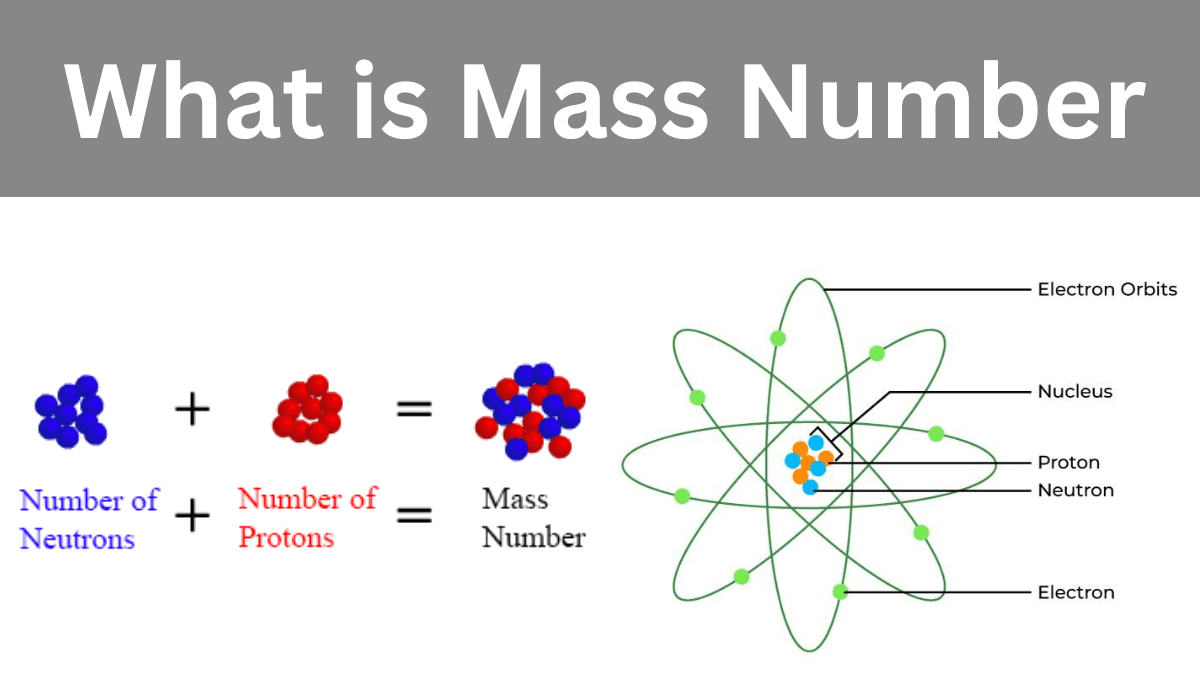
The mass number refers to the total number of protons and neutrons in an atom’s nucleus. It is basically a measurement of the entire mass of the nucleus.
An atom’s mass number represents the total number of protons and neutrons in its nucleus. It is frequently represented by the sign “A.” For example, if an atom contains 6 protons and 6 neutrons, its mass number is 12. The mass number contributes to an element’s total mass, hence determining its atomic mass.
Because it makes it easier to discriminate between various isotopes of an element, the mass number is significant. Atoms of the same element that differ in the number of neutrons but have the same number of protons are called isotopes. For example, the mass number of carbon-14 is 14 because it has 6 protons and 8 neutrons, whereas the mass number of carbon-12 is 12 because it contains 6 protons and 6 What is Mass Number neutrons.
So friends, what will happen if you know about what is mass number? Now we will tell you about the definition of mass number, which is written just below it. You can get more information by reading its definition.
Defination of Mass Number
An atom’s mass number refers to the total number of protons and neutrons in its nucleus. It effectively measures the overall mass of the nucleus. Carbon-12 has a mass number of 12 because its nucleus contains 6 protons and 6 neutrons. Carbon-14 has a mass of 14 because it has 6 protons and 8 neutrons. The mass number distinguishes various isotopes of an element.An atom’s mass number refers to the total number of protons and neutrons in its nucleus.
So friends, this was the definition of mass number in which you would have got more information about mass number. Number from the mind is a number used in What is Mass Number chemistry from which many values can be derived.
Example of Mass Number
So friends, there is an exam for mass number also, you can read it below. We have written below for you on the exam for mass number, after which you will be able to get more information about mass number.
Consider carbon-12. The number 12 denotes carbon-12’s mass number. This indicates that a carbon-12 atom has 6 protons (since carbon has an atomic number of 6) and 6 neutrons (because 12 – 6 = 6).
Friends, now you must have got to know about the example of mass number, after this you must have got more information about mass number.
Difference Between Atomic & Mass Numbers
Atomic Number (Z) refers to the number of protons in an atom’s nucleus. It determines an element’s identification. For example, all carbon atoms contain 6 protons in their nucleus, hence the atomic number of carbon (C) is 6.
The mass number (A) refers to the total number of protons and neutrons in an atom’s nucleus. It provides an approximate measurement of an atom’s mass. The ‘A’ sign represents the mass number. Carbon-12, for example, contains six protons and six neutrons, giving it a mass of twelve.
Conclusion
So friends, today we told you in this post what is meat number, what is its definition and what are its examples. We have given you all the information in this post. If any other person like you also needs the same information, then definitely share this post. Thanks for completing the post